What is Markovnikov’s Rule?
Markovnikov’s Rule, also known as Markownikoff’s rule, can be used to describe the outcome of some chemical addition reactions. The Russain chemist Vladimir Vasilyevich Markovnikov first formulated this rule in 1865.
What is Markovnikov’s Rule?
When a protic acid (HX) is added to an asymmetric alkene, the acidic hydrogen attaches itself to the carbon having a greater number of hydrogen substituents whereas the halide group attaches itself to the carbon atom which has a greater number of alkyl substituents.
To simplify the rule, it can also be stated as – “Hydrogen is added to the carbon with the most hydrogens and the halide is added to the carbon with least hydrogens”.
An example of a reaction that observes Markovnikov’s rule is the addition of hydrobomicacid (HBr) to propene, which is shown below.
An example of a reaction that observes Markovnikov’s rule is the addition of hydrobomicacid (HBr) to propene, which is shown below.
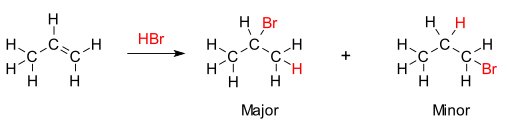
Markovnikov’s rule
It can be observed from the reaction illustrated above that the majority of the product formed obeys Markovnikov’s rule, whereas the minority of the product does not.
Let us consider the addition reaction wherein an alkene reacts with water to give rise to an alcohol. This reaction proceeds via the formation of a carbocation. It is observed in this reaction that the hydroxyl group attaches itself to the carbon with more carbon-carbon bonds whereas the hydrogen atom attaches itself to the other carbon in the double bond which has more carbon-hydrogen bonds.
Let us consider the addition reaction wherein an alkene reacts with water to give rise to an alcohol. This reaction proceeds via the formation of a carbocation. It is observed in this reaction that the hydroxyl group attaches itself to the carbon with more carbon-carbon bonds whereas the hydrogen atom attaches itself to the other carbon in the double bond which has more carbon-hydrogen bonds.
What is the Mechanism Behind Markovnikov’s Rule?
To understand this mechanism, let us consider the same example illustrated earlier, i.e. the addition reaction of hydrobromic acid with propene. The Mechanism of Markovnikov’s rule can be broken down into the following two steps.
Step 1
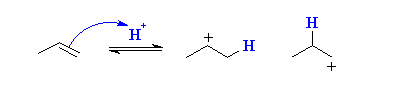
The Mechanism behind Markovnikov’s Rule (Step 1)
The alkene is protonated and it gives rise to the more stable carbocation as shown below.
From the illustration shown above, we can see that there are two types of carbocations that can be formed from the protonation of the alkene, one is a primary carbocation and the other is a secondary carbocation. However, the secondary carbocation is far more stable and therefore, its formation is preferred over the formation of a primary carbocation.
Step 2
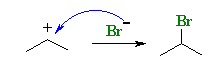
The Mechanism behind Markovnikov’s Rule (Step 2)
The halide ion nuceleon now attacks the carbocation. This reaction yields the alkyl halide. Since the formation of the secondary carbocation is preferred, the major product of this reaction would be 2-bromopropane as illustrated below.
It is important to note that the Markovnikov’s rule was developed specifically for its application in the addition reaction of hydrogen halides to alkenes. The opposite of ‘Markovnikov’ addition reactions can be described as Anti markovinico based on the regioselectivity of the reaction.
Solved Exercises on Markovnikov’s Rule
What is the Reasoning behind Markovnikov’s Rule?
The protonation of the alkene by the protic acid results in the formation of a carbocation. The most stable carbocation is the one in which the positive charge is held by the carbon with the greatest number of alkyl substituents. Therefore, the majority of the product features the addition of the halide to the carbon having fewer hydrogen substituents.
Does the Following Reaction obey Markovnikov’s Rule?

Since the bromine atom attaches itself to the carbon having a greater number of hydrogen atoms (or fewer alkyl substituents), the reaction illustrated above does not obey Markovnikov’s rule.
If the Following Reaction obeys Markovnikov’s Rule, What would be the Major Product?

The product in which bromine is attached to the secondary carbon would be the major product. The CH3CH=CHBr product would be the minor product as per Markovnikov’s Rule.
Comments
Post a Comment
Thank You For Visit To My Blog ...
you will soon get the reply.. for your comment.......