What is a Chemical Reaction?
What is a Chemical Reaction?
A chemical reaction is in which the bonds are broken within reactant molecules, and new bonds are formed within product molecules in order to form a new substance.
Chemical reactions are all around us, from the metabolism of food in our body to how the light we get from the sun is the results of chemical reactions. Before beginning with chemical reactions, it is important to know about physical and chemical change.

Chemicals Reactions
A burning candle is the best example of physical and chemical change. Take a candle and light it. As time passes, we can observe that the candle changes to wax. If you cover the candle with a jar, it will extinguish.
In the demonstration, burning of the candle is a chemical change while conversion of the candle to wax is a physical change. In a physical change, there is basically a change of state of the substance but in the case of a chemical change mostly a new substance is formed in which either energy is given off or absorbed. Thus we can conclude that chemical changes are accompanied by certain physical changes.
Basic Concepts of Chemical Reactions
- A Chemical Reaction is a process that occurs when two or more molecules interact to form a new product(s).
- Compounds that interact to produce new compounds are called reactants whereas the newly formed compounds are called products.
- Chemical reactions play an integral role in different industries, customs and even in our daily life. They are continuously happening in our general surroundings; for example, rusting of iron, pottery, fermentation of wine and so on.
- In a chemical reaction, a chemical change must occur which is generally observed with physical changes like precipitation, heat production, colour change etc.
- A reaction can take place between two atoms or ions or molecules and they form a new bond and no atom is destroyed or created but a new product is formed from reactants.
- The rate of reaction depends on and is affected by factors like pressure, temperature, the concentration of reactants.
Recommended Videos
Chemical Equations
Due to the vast amounts of chemical reactions happening around us, a nomenclature was developed to simplify how we express a chemical reaction in the form of a chemical equation. A chemical equation is nothing but a mathematical statement which symbolizes the product formation from reactants while stating certain condition for which how the reaction has been conducted.
The reactants are on the left-hand side whereas products formed on the right-hand side connected by a one-headed or two-headed arrows. For example, a reaction
A + B → C + D
Here, A and B are the reactants, which react to form the products C and D. In an actual chemical equation, reactants are denoted by their chemical formula. In order to assure the law of conservation of mass, a chemical equation must be balanced i.e. the number of atoms on both sides must be equal. This is the balancing of the equation.
Let us consider an actual chemical reaction between Methane(CH₄) and Oxygen (O2),
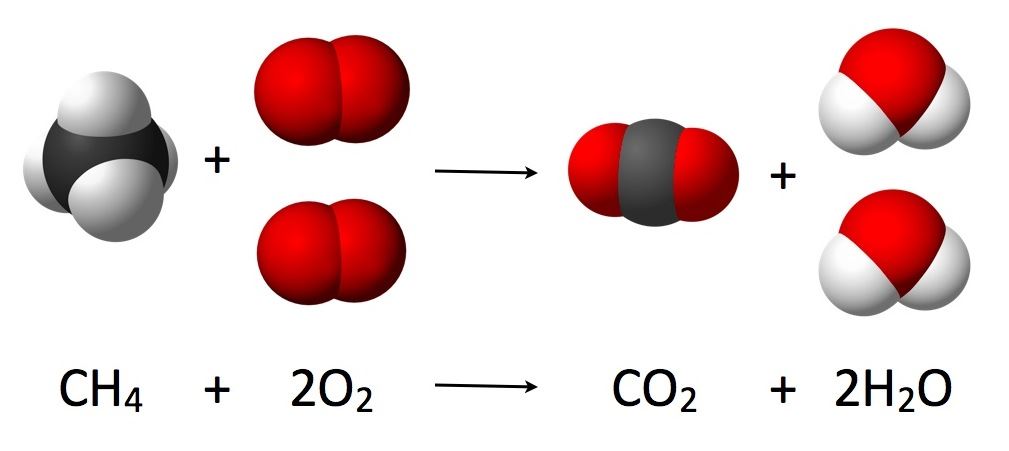
Combustion Chemical Reaction
Here we can see how the number of each atom on the left side is balanced on the right side, as stated by the law of conservation of mass.
Types of Chemical Reactions
The basis for different types of reactions is the product formed, the changes that occur, the reactants involved and so on. Different types of reactions are
- Synthesis reaction
1. Combustion Reaction
A combustion reaction is a reaction with a combustible material with an oxidizer to give an oxidized product. An oxidizer is a chemical a fuel requires to burn, generally oxygen. Consider the example of combustion of magnesium metal.
Here, 2 magnesium atoms react with a molecule of oxygen producing 2 molecules of the compound magnesium oxide releasing some heat in the process.
2. Decomposition Reaction
A Decomposition reaction is a reaction in which a single component breaks down into multiple products. Certain changes in energy in the environment have to be made like heat, light or electricity breaking bonds of the compound. Consider the example of the decomposition of calcium carbonate giving out CaO (Quick Lime) which is a major component of cement.
Here, the compound Calcium carbonate when heated breaks down into Calcium Oxide and Carbon Dioxide.
3. Neutralization Reaction
A Neutralization reaction is basically the reaction between an acid and a base giving salt and water as the products. The water molecule formed is by the combination of OH– ions and H+ ions. The overall pH of the products when a strong acid and a strong base undergo a neutralization reaction will be 7. Consider the example of the neutralization reaction between Hydrochloric acid and Sodium Hydroxide giving out sodium chloride(Common Salt) and water.
Here, an acid and a base, Hydrochloric acid and Sodium Hydroxide react in a neutralization reaction to produce Sodium Chloride(Common Salt) and Water as the products.
4. Redox Reaction
A REDuction-OXidation reaction is a reaction in which there is a transfer of electrons between chemical species. Let us consider the example of an electrochemical cell-like redox reaction between Zinc and Hydrogen.
Here, A Zinc atom reacts with 2 ions of positively charged hydrogen to which electrons get transferred from zinc atom and hydrogen becomes a stable molecule and Zinc ion is the product.
5. Precipitation or Double-Displacement Reaction
It is a type of displacement reaction in which two compounds react and consequently, their anions and cations switch places forming two new products. Consider the example of the reaction between silver nitrate and sodium chloride. The products will be silver chloride and sodium nitrate after the double-displacement reaction.
Here, Silver Nitrate and Sodium Chloride undergo a double displacement reaction. Wherein Silver replaces Sodium in Sodium Chloride and Sodium joins with Nitrate becoming Sodium Nitrate along with the Silver Chloride as the product.
6. Synthesis Reaction
A Synthesis reaction is one of the most basic types of reaction wherein multiple simple compounds combine under certain physical conditions giving out a complex product. The product will always be a compound. Let us consider the Synthesis reaction of sodium chloride with reactants solid sodium and chloride gas.
Here, we have 2 Atoms of solid Sodium reacting with Chlorine gas giving out Sodium Chloride viz. Common Salt as the product.
Important Points to Remember
- In a chemical change, a new compound is formed but in a physical change, the substance changes its state of existence.
- Atoms or ions or molecules which react to form a new substance are called reactants; the new atoms or molecules formed are products.
- A chemical reaction follows the law of conservation of mass. That is no atom is destroyed or created but only a new product is formed from reactants.
Comments
Post a Comment
Thank You For Visit To My Blog ...
you will soon get the reply.. for your comment.......