What is Gay-Lussac’s Law?
What is Gay-Lussac’s Law?
Gay-Lussac’s law is a gas law which states that the pressure exerted by a gas (of a given mass and kept at a constant volume) varies directly with the absolute temperature of the gas. In other words, the pressure exerted by a gas is proportional to the temperature of the gas when the mass is fixed and the volume is constant.
This law was formulated by the French chemist Joseph Gay-Lussac in the year 1808. The mathematical expression of Gay-Lussac’s law can be written as follows:
P ∝ T ; P/T = k
Where:
- P is the pressure exerted by the gas
- T is the absolute temperature of the gas
- k is a constant.
The relationship between the pressure and absolute temperature of a given mass of gas (at constant volume) can be illustrated graphically as follows.

From the graph, it can be understood that the pressure of a gas (kept at constant volume) reduces constantly as it is cooled until the gas eventually undergoes condensation and becomes a liquid.
Formula and Derivation
Gay-Lussac’s law implies that the ratio of the initial pressure and temperature is equal to the ratio of the final pressure and temperature for a gas of a fixed mass kept at a constant volume. This formula can be expressed as follows:
(P1/T1) = (P2/T2)
Where:
- P1 is the initial pressure
- T1 is the initial temperature
- P2 is the final pressure
- T2 is the final temperature
This expression can be derived from the pressure-temperature proportionality for gas. Since P ∝ T for gases of fixed mass kept at constant volume:
P1/T1 = k (initial pressure/ initial temperature = constant)
P2/T2 = k (final pressure/ final temperature = constant)
Therefore, P1/T1 = P2/T2 = k
Or, P1T2 = P2T1
Examples of Gay-Lussac’s Law
When a pressurized aerosol can (such as a deodorant can or a spray-paint can) is heated, the resulting increase in the pressure exerted by the gases on the container (owing to Gay-Lussac’s law) can result in an explosion. This is the reason why many pressurized containers have warning labels stating that the container must be kept away from fire and stored in a cool environment.
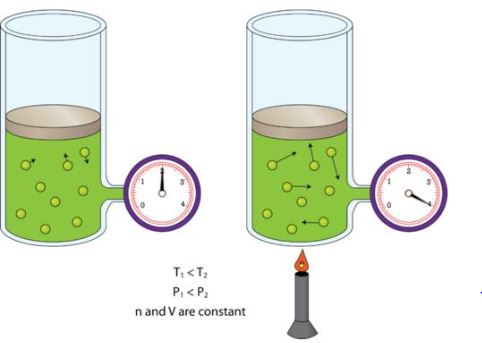
An illustration describing the increase in pressure which accompanies an increase in the absolute temperature of a gas kept at a constant volume is provided above. Another example of Gay-Lussac’s law can be observed in pressure cookers. When the cooker is heated, the pressure exerted by the steam inside the container increases. The high temperature and pressure inside the container cause the food to cook faster.
Solved Exercises on Gay-Lussac’s Law
Exercise 1
The pressure of a gas in a cylinder when it is heated to a temperature of 250K is 1.5 atm. What was the initial temperature of the gas if its initial pressure was 1 atm.
Given,
Initial pressure, P1 = 1 atm
Final pressure, P2 = 1.5 atm
Final temperature, T2 = 250 K
As per Gay-Lussac’s Law, P1T2 = P2T1
Therefore, T1 = (P1T2)/P1 = (1*250)/(1.5) = 166.66 Kelvin.
Exercise 2
At a temperature of 300 K, the pressure of the gas in a deodorant can is 3 atm. Calculate the pressure of the gas when it is heated to 900 K.
Initial pressure, P1 = 3 atm
Initial temperature, T1 = 300K
Final temperature, T2 = 900 K
Therefore, final pressure (P2) = (P1T2)/T1 = (3 atm*900K)/300K = 9 atm.
Comments
Post a Comment
Thank You For Visit To My Blog ...
you will soon get the reply.. for your comment.......